Leaphy 1A
Leaphy, the single trusted source for information about medicines and more
€131,700
total amount raised in round
163%
- Backed by over 70 investors
- Eligible for a tax reduction
Campaign Closed
Introduction Market analysis
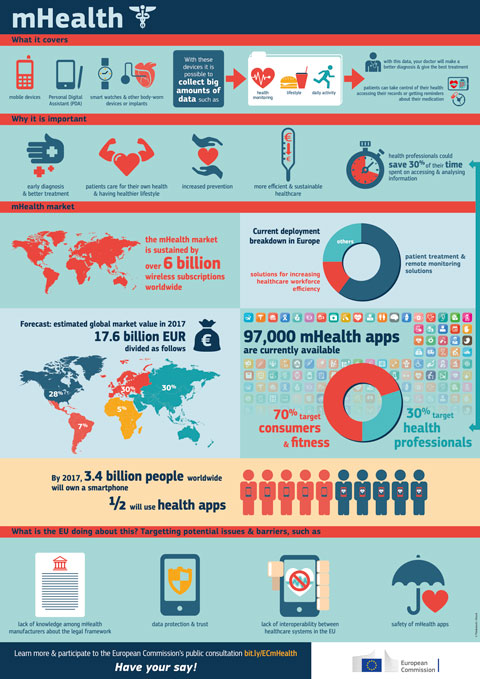
Today, patients complain about the readability of the paper patient information leaflets (PIL) and both patient organisations and the pharmaceutical industry would rather like to work with online leaflets (ePIL). Recent reports from European Commission and the European Medicines Agency
(EMA) have recommended to explore the use of electronic media to provide the information included in PIL and to consider opportunities which can improve their readability.
(EMA) have recommended to explore the use of electronic media to provide the information included in PIL and to consider opportunities which can improve their readability.
eLeaflets (Leaphy's core value proposition)
- Fact 1: environmental factor: In Europe, around 32 billion (18 billion prescription medicines, 14 billion OTC medicines) packs of medicines are sold annually, which is equal to the amount of paper versions of the PIL which are inserted i or attached to the outer packaging of medicinal products. Let's assume that 1 PIL in length is on average 2 (recto-versio) printed pages, the number of pages printed for a paper PIL is 64 billion pages, which is almost half the internet in 14 pages (in March 2015). Converted into number of trees (average 1 tree per 8,500 pages), it requires around 7.5 million trees every year to print these PILs in Europe.
- Fact 2: ePIL lobby: Pharmaceutical companies are currently lobbying to abandon the obligation of paper PILs. By offering the ePIL database at very low cost, companies have an incentive to use Leaphy as a lobby tool to pursue a legislative change in the EU pharma legislation.
- Fact 3: compulsory use of 2D matrix code: The Falsified Medicines Directive foresees that in principle all prescription medicines shall bear a data matrix on their outer packaging. This means that 75% of medicines registered in Europe (or 375,000 registered medicines) will have a harmonised QR code on their outer packaging which patients could scan to refer them to an ePIL.
- Fact 4: Tamper verification features prevent pharmacists from opening medicinal packs: The Falsified Medicines Directive foresees that each pack of prescription medicines shall bear a tamper verification feature which seals the outer packaging. As such, pharmacists will not be able to open the package to take out the leaflet for educational purposes towards patients at the counter of the pharmacy. Healthcare professionals can interface with Leaphy for direct access to PILs.
Interface with Leaphy by healthcare professionals
Hospitals, pharmacists, doctors and home-care organisations have showed interest in interfacing with the leaflets' database directly in order to have immediate access to the latest product information in an easy to use format. The company has signed its first API contract with Wit-Geel Kruis and discussions with several hospital groups and companies supporting pharmacy web-shops are ongoing.
Competition
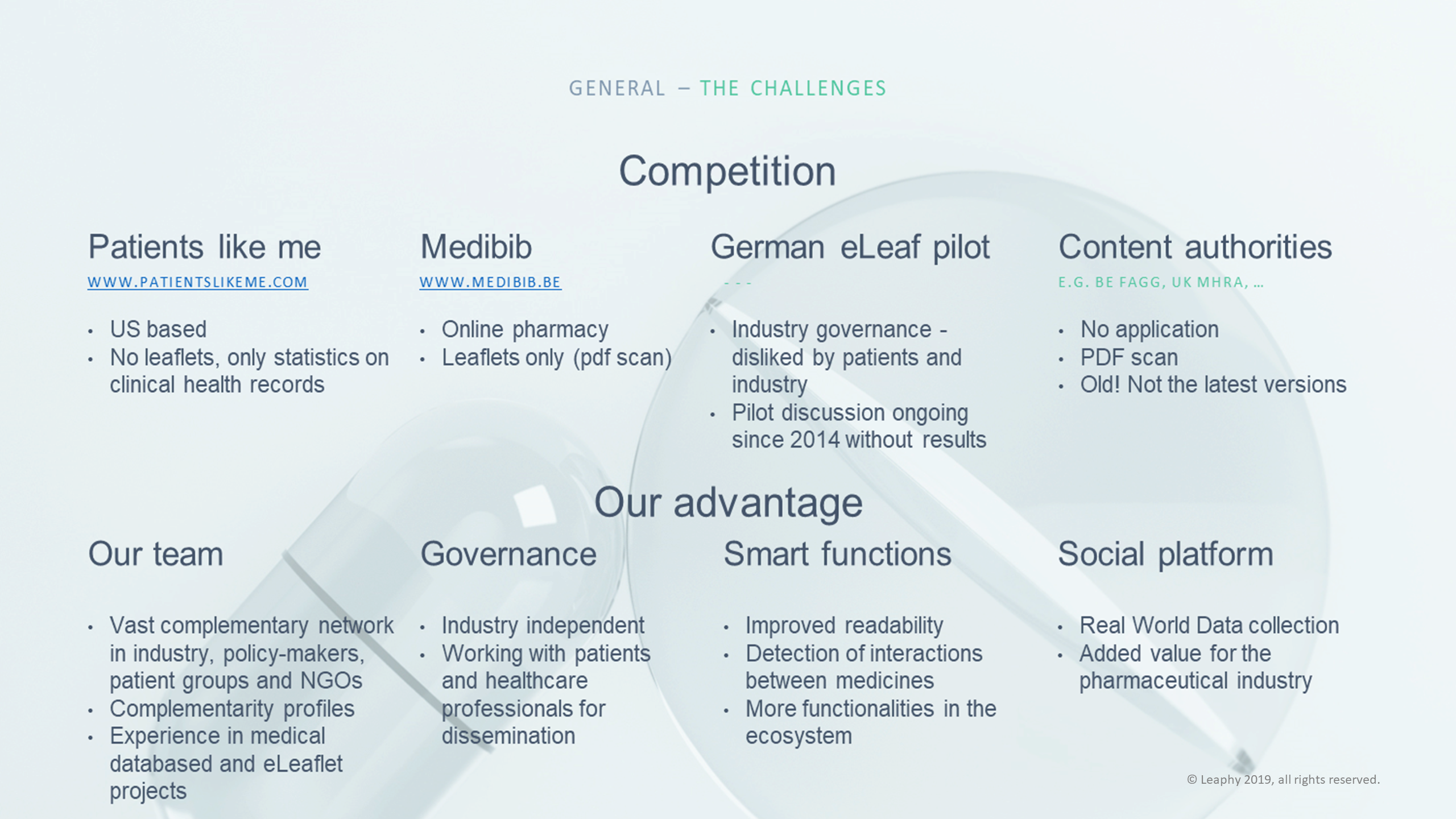
- German e-leaflet pilot: German industry stakeholders have been working on a pilot since 2014. However, the project today has governance problems since much time has been lost due to discussions on the cost allocation of the project. In addition, the scope is limited to Germany.
- e-compendium - Pharma.be: the Belgian innovative industry association is running the e-compendium. The platform however is only web-based and only members of the association are allowed to publish their leaflets. As such, a big piece of the market is missing. In addition, the costs for companies to publish their leaflets on e-compendium is nearly a 10-fold compared to the price strategy of Leaphy. Pharma.be has filed a court case against Leaphy to block competition. It has lost the case in a summary proceedings.
- Medibib: Medibib key business is a web-based online pharmacy. The PILs are only made available in PDF format. There are no patient services offered.
- National medicines agencies: competent authorities have the obligation to publish PILs online. They have however no further interests in exploring features that go beyond their legal obligations. In addition, Leaphy's research has shown that authorities are often late in publishing the latest updates (up to 1 year of delay).
- Patients Like Me: is a US-based platform in which patients can input their health data for the purpose of creating statistics. They do not offer PILs or any other information about medicinal products.